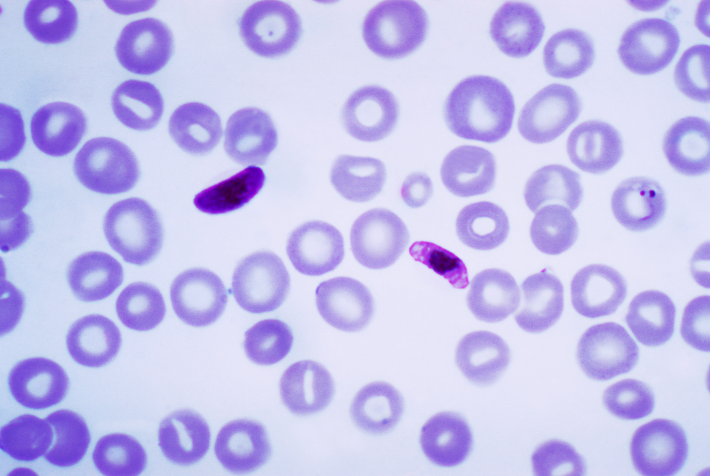
One of the most common and fatal infectious illnesses in the world is malaria. New chemicals are always needed because of the possibility that malaria parasites will develop resistance to the present medications. Under the direction of Prof. Dr. Svetlana B. Tsogoeva, a group of researchers at FAU (Friedrich Alexander Universität, Erlangen Nürnberg, Germany) have recently combined the anti-malaria medication artemisinin with coumarin, which is also present in plants, to create an autofluorescent molecule. This autofluorescence is especially useful because it may be utilized for imaging in living cells and displays the medication’s action in a precise time sequence. The working group additionally discovered that Plasmodium falciparum, a drug-resistant malaria parasite, can be eliminated by the auto-fluorescent artemisinin-coumarin hybrids.
They have published the findings in the journal Chemical Science.
Artemisinin, derived from the plant known as sweet wormwood (Artemisia annua L.), is a widely used and extremely effective component in malaria medications. A secondary plant component present in a variety of plants is called coumarin. When developing anti-malaria medications, active ingredients are marked with fluorescent compounds, so imaging methods may precisely track their sequential action against malaria infections. Artemisinin has already been labelled with this fluorescent method.
Combination of substances for autofluorescence
Labelling with fluorescent materials has the major drawback of changing the drug’s mechanism of action. This might indicate, for instance, that following fluorescent tagging, malaria-infected cells absorb particular drugs, such as artemisinin, in a different way than they did before. The medication’s solubility may also alter. Autofluorescent hybrids, which are substances composed of two or more basic chemicals that are intrinsically fluorescent and whose mode of action can be accurately examined using imaging techniques, prevented this from happening.
Also Read| Researchers discovered a suite of genes that influence the human head shape
Active agent with special skills
The group at the Chair of Organic Chemistry, under the direction of Professor Tsogoeva, made the decision to combine bioactive coumarins with artemisinin due to the anti-malaria characteristics of coumarin derivatives. Additionally, it is simple to chemically change them to make them incredibly luminous. Not only was it feasible to see this first autofluorescent artemisinin-coumarin hybrid’s method of action in living, P. falciparum-infected red blood cells, the researchers found. Along with Dr. Diogo R. M. Moreira (Instituto Gonçalo Moniz, Fiocruz, Bahia, Brazil) and Prof. Barbara Kappes (Department of Chemical and Biological Engineering, FAU), they also found that the active agent was highly effective against P. falciparum strains that are resistant to chloroquin and other malaria medications in vitro (in a test tube). Above all, however, the new compound also proved highly effective against malaria pathogens in vivo in mouse models.
The researchers at FAU believe that by developing the first autofluorescent artemisinin-coumarin hybrid, they have made significant progress toward combating multi-drug resistance in malaria treatment and set the stage for the future development of other autofluorescent agents.
Journal Reference: Herrmann L, Leidenberger M, de Morais AS, Mai C, Çapcı A, Silva MD, Plass F, Kahnt A, Moreira DR, Kappes B, Tsogoeva SB. Autofluorescent antimalarials by hybridization of artemisinin and coumarin: In vitro/in vivo studies and live-cell imaging. Chemical Science. 2023. DOI: https://doi.org/10.1039/D3SC03661H.
Source: FAU News
Report: Achuth B S
Last updated: