Researchers at the University of Stuttgart have used “DNA origami” to successfully manipulate the composition and functionality of biological membranes. They created a technique that might make it easier to deliver heavy therapeutic doses into cells. This makes it possible to provide medication and other therapeutic measures in a more targeted manner. Their findings were published in the journal Nature Materials.
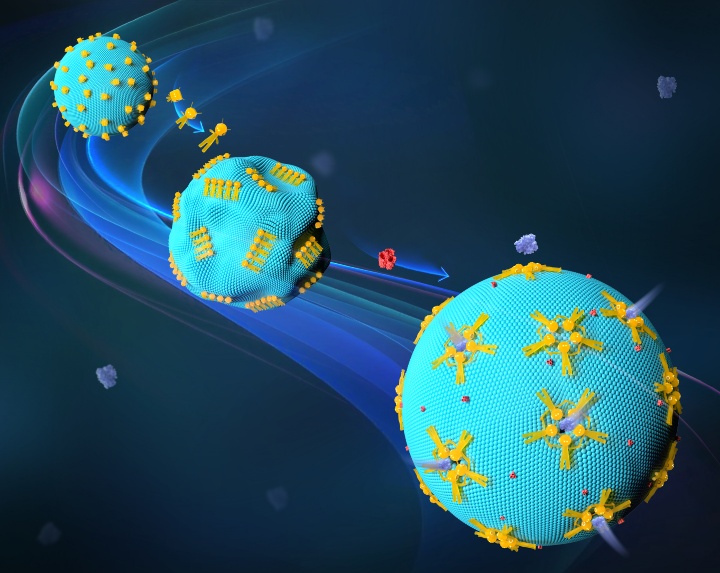
A cell’s morphology and form are crucial to its biological function. This is in line with the “form follows function” theory that is prevalent in contemporary architecture and design. In synthetic biology, it can be difficult to apply this idea to artificial cells. Promising solutions are now available thanks to developments in DNA nanotechnology. They make it possible to design new transport channels that are sufficiently big to help therapeutic proteins flow through cell membranes. In this new area, researchers like Prof. Laura Na Liu, a fellow at the Max Planck Institute for Solid State Research (MPI-FKF) and director of the University of Stuttgart’s 2nd Physics Institute, have created a novel tool for manipulating the permeability and form of lipid membranes in artificial cells. These membranes are simplified representations of biological membranes composed of lipid bilayers enclosing an aqueous compartment. They are helpful for researching lipid behavior, protein interactions, and membrane dynamics.
The development of useful synthetic cells could be facilitated by this novel instrument. The goal of Laura Na Liu’s scientific effort is to have a big impact on the study and creation of novel treatments. Liu and her group have been successful in enabling programmable interactions with synthetic cells through the use of signal-dependent DNA nanorobots. “This work is a milestone in the application of DNA nanotechnology to regulate cell behavior,” Liu stated. The group works with simple, cell-sized constructs called giant unilamellar vesicles (GUVs), which resemble live cells. The researchers were able to modify the form and function of these artificial cells by using DNA nanorobots.
One of Laura Na Liu’s primary areas of study is DNA nanotechnology. She specializes in DNA origami constructions, which are DNA strands folded using shorter, specially crafted DNA sequences known as staples. As reconfigurable nanorobots that can reversibly change their shape and affect their immediate environment in the micrometer range, the Liu team employed DNA origami constructs. The researchers discovered that the deformation of the GUVs and the creation of artificial channels in the model GUV membranes can be connected to the transformation of these DNA nanorobots. Large molecules were able to flow through the membrane thanks to these channels, which can be sealed again if needed.
This means that we can use DNA nanorobots to design the shape and configuration of GUVs to enable the formation of transport channels in the membrane,
It is extremely exciting that the functional mechanism of the DNA nanorobots on GUVs has no direct biological equivalent in living cells.
Stephan Nussberger
The new study poses fresh queries, such as whether synthetic platforms, such DNA nanorobots, could be made simpler than their biological counterparts while yet operating in a biological setting.
An crucial step in this direction is the latest study. Certain compounds and substances can enter cells efficiently thanks to the cross-membrane channel system that DNA nanorobots have constructed. Above all, these channels are sizable and have the ability to be configured to shut off when necessary. This technology has the potential to help deliver therapeutic proteins or enzymes to their intended locations within living cells. As a result, it presents fresh opportunities for drug administration and other therapeutic approaches.
Our approach opens up new possibilities to mimic the behavior of living cells. This progress could be crucial for future therapeutic strategies.
Hao Yan
Also Read: How the molecular dynamics of CAR T cells influence their ability to cancer-killing behavior
One of the study’s primary authors, Laura Na Liu, is the Director of the University of Stuttgart’s 2nd Physics Institute and a Fellow at the Max Planck Institute for Solid State Research (MPI-FKF). Members of the interdisciplinary research team come from a number of University of Stuttgart institutes, including the Institute of Theoretical Physics IV, the Department of Biophysics at the Institute of Biomaterials and Biomolecular Systems, and the 2nd Physics Institute. The Alexander von Humboldt Research Award laureate Prof. Hao Yan is also participating in the investigation. He is conducting research at Arizona State University’s Biodesign Center for Molecular Design and Biomimetics while being hosted by Liu’s team.
Source: University of Stuttgart – News
Journal Reference: Fan, Sisi, et al. “Morphology Remodelling and Membrane Channel Formation in Synthetic Cells via Reconfigurable DNA Nanorafts.” Nature Materials, 2025, pp. 1-9, DOI: https://doi.org/10.1038/s41563-024-02075-9.
Last Modified: