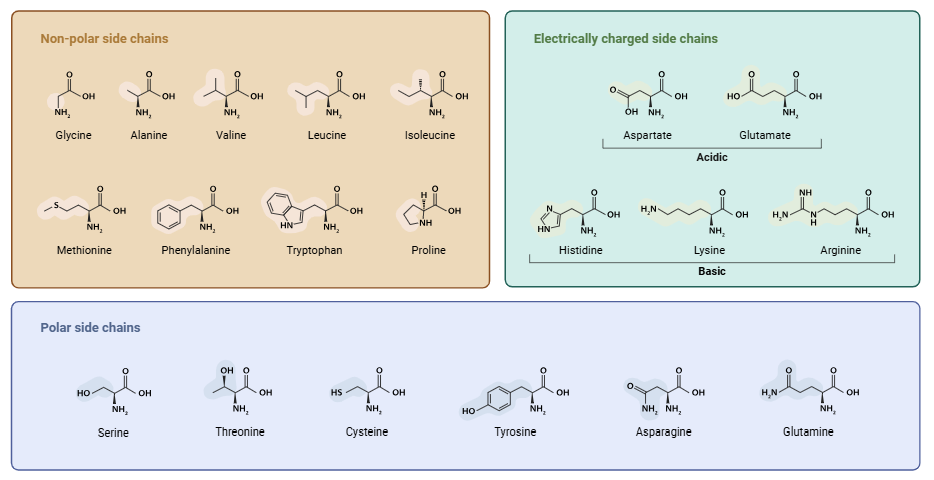
Amino acids are the basic structural units of proteins, which are critical macromolecules of all living organisms. Proteins play a role in almost every aspect of biological function, and structure and function are conferred by the sequence and nature of amino acids. Read the detailed description of amino acids.
Structure of Amino Acids
All amino acids have a central (alpha) carbon atom that is attached to four different groups.
- An amino group (–NH₂)
- A carboxyl group (–COOH)
- A hydrogen atom (–H)
- A variable side chain, known as the R group

The R group varies for different amino acids and imparts its specific chemical and physical characteristics. The genetic code encodes 20 standard amino acids, and they can be grouped according to the character of their side chains.
Classification of Amino Acids According to Side Chains
- Non-Polar (Hydrophobic)
- Polar Uncharged
- Polar Charged (Positive or Negative)
Non-Polar / Hydrophobic Amino Acids
These amino acids possess hydrophobic side chains, which are often involved in maintaining protein structure by contributing to the protein core.
Glycine: Code (Gly, G)
- Smallest amino acid
- Side chain: Hydrogen atom
- Achiral
- Contributes flexibility, commonly seen in protein turns.
Alanine: Code (Ala, A)
- Side chain: Methyl group (–CH₃)
- Stabilizes α-helix structures.
Valine: Code (Val, V)
- Side chain is isopropyl group (—CH(CH3)2)
- The condition called sickle cell anaemia is due to the replacement of normal glutamic acid by valine in the 6th position of the haemoglobin protein chain.
Leucine: Code (Leu, L)
- Side chain: Isobutyl group
- Branched-chain amino acid.
Isoleucine: Code (Ile, I)
- Side chain: Secondary butyl group.
- Both α and β carbons are chiral, can form diastereomers.
- One of the more hydrophobic amino acids.
Methionine: Code (Met, M)
- Side chain: Thioether (sulfur-containing).
- Start codon for protein synthesis (AUG).
Proline: Code (Pro, P)
- Secondary amine (cyclic structure: pyrrolidine ring).
- Induces kinks or turns in protein chains.
- Commonly found in collagen, hydroxyproline is a more stable derivative.
- Referred to as a helix breaker because of its rigid conformation.
Phenylalanine: Code (Phe, F)
- Aromatic amino acid.
- Side chain: Phenyl group
- Absorbs UV light; hydrophobicity of aromatic amino acids.
Tryptophan: Code (Trp, W)
- Side chain: Indole ring (largest R group).
- Strong UV absorption (~280 nm).
Tyrosine: Code (Tyr, Y)
- Side chain: Phenol group.
- More hydrophilic owing to –OH group.
- UV absorbing characteristics.
Polar Uncharged Amino Acids
These amino acids are capable of forming hydrogen bonds and participate in a number of metabolic pathways.
Serine: Code (Ser, S)
- Side chain: Hydroxymethyl group.
- Common phosphorylation site.
- Precursor to selenocysteine.
Threonine: Code (Thr, T)
- Side chain: Ethanol group.
- Can form diastereomers.
- Site for phosphorylation.
Asparagine: Code (Asn, N)
- Side chain: Carboxamide
- Involved in N-linked glycosylation.
Glutamine: Code (Gln, Q)
- Side chain: Ethyl amide group.
- Involved in nitrogen metabolism.
Cysteine: Code (Cys, C)
- Side chain: Sulfhydryl (–SH).
- Forms disulfide bonds, essential for protein structure.
Polar Charged Amino Acids
Positively charged
Lysine: Code (Lys, K)
- Side chain is called as butyl ammonium.
- Basic amino acid.
- An amino acid formed by modifying lysine is called Pyrrolysine.
Arginine: Code (Arg, (R)
- Side chain is called guanidino.
- Basic amino acid.
Histidine: Code (His, H)
- Has an imidazole group side chain.
- It is positively charged at physiological pH.
Negatively charged
Aspartic Acid: Code (Asp, D)
- Side chain is β β-carboxylic group.
- Acidic amino acid.
Glutamic Acid: Code (Glu, E)
- Side chain is ɣ carboxylic acid.
- Acidic amino acid.
- It is a neurotransmitter.
Amino acids can be classified as Ketogenic , glucogenic amino acid or both ketogenic and glucogenic.
- Ketogenic Amino Acid: The carbon skeleton of these amino acids gets converted into ketone body precursors such as acetyl CoA. Those amino acids are Lysine and Leucine.
- Glucogenic Amino Acid: The carbon skeleton of these amino acids gets converted into glucose precursors such as pyruvate. Those amino acids are glycine, alanine, valine, methionine, proline, serine, asparagine, glutamine, cysteine, histidine, aspartate, glutamate, arginine.
- Both ketogenic and glucogenic amino acids: Tryptophan, tyrosine, phenylalanine, threonine, and isoleucine.
Last Modified:
Qualified CSIR - National Eligibility Test (NET), eligible for Assistant Professorship in any Indian university. Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the fourth rank. Conducted various seminars and presentations. Experienced in ELISA, Blotting, and other Good Laboratory Practices. Attended a certificate course in Patent Analytics. Done 6 months of internship in ICMR - Vector Control Research Center, Puducherry. 3 years of tutoring experience.