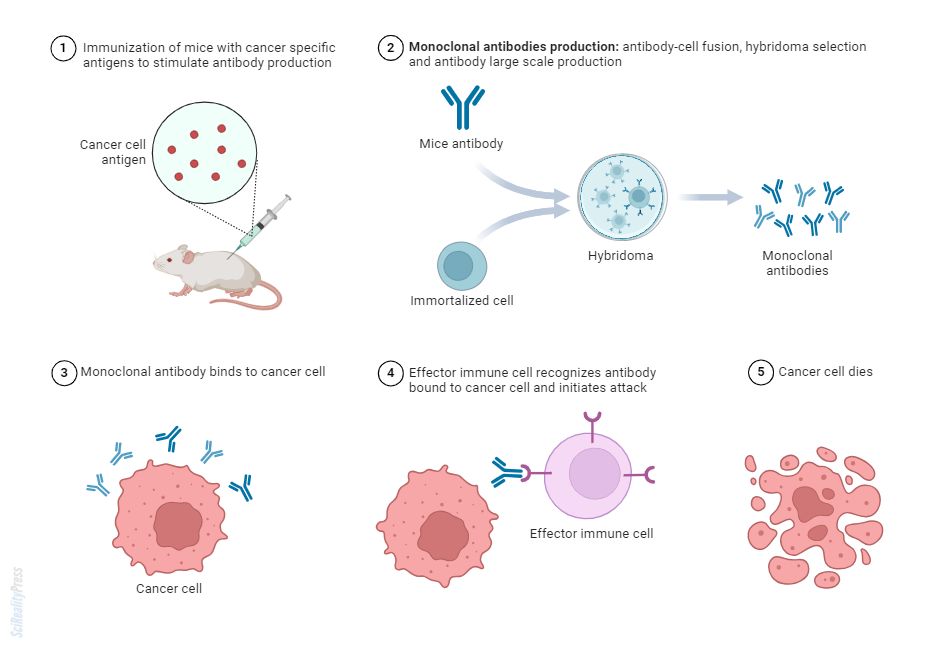
Monoclonal antibodies revolutionized medicine by introducing a range of targeted therapies against malignant diseases, autoimmune disorders, infectious diseases, and many others. They are engineered from a single clone of cells to bind to antigens with very high precision. This article presents the underlying science behind monoclonal antibodies, including their applications, development process, and future potential.
What Are Monoclonal Antibodies?
Monoclonal antibodies are lab-produced molecules that aim to take the place of natural antibodies. As the offspring of a single clone of cells, they will replicate themselves and act like natural antibodies to regain balance in the immune system’s fighting of viruses, bacteria, cancer cells, or any other foreign entities entering the body. In contrast with polyclonal antibodies, which contain several antibodies produced by different immune cells, monoclonal antibodies are identical and have only one specific antigen.
The concept of monoclonal antibodies dates as far back as the 1970s when César Milstein and Georges Köhler discovered the hybridoma technique, a method for producing large quantities of identical antibodies. This finding won them the Nobel Prize in Physiology or Medicine in 1984. Thus, with advancements in biotechnology, the production, efficacy, and safety of mAbs have improved since then.
How Monoclonal Antibodies Work
Monoclonal antibodies are only able to bind with specific antigens, which are unique molecules on the surface of the pathogen or abnormal cells. That binding can neutralize a pathogen directly, tag it for removal by other components of the immune system, or block the signals that proliferate or survive cancer cells.
Mechanisms
- Antibody-Dependent Cellular Cytotoxicity (ADCC): mAbs can engage immune cells, like natural killer cells, to kill the target cell.
- Complement-Dependent Cytotoxicity (CDC): mAbs can activate the complement system, which will then lyse the target cell.
- Neutralization: mAbs can directly neutralize either toxins or pathogens by binding and subsequently blocking their entrance into host cells.
- Opsonization: The interaction of mAb with the pathogen can promote the recognition and uptake of the pathogen by phagocytes, thereby leading to its destruction.
- Signal Blockade: mAbs can block the receptors on cells from receiving growth or survival signals, a mechanism very useful in cancer therapy.
Structure
Monoclonal antibodies are Y-shaped proteins comprising four polypeptide chains, two heavy and two light chains. These chains are linked by disulfide bonds to yield a quaternary structure with definite regions that carry out specific functions.

1. Heavy Chains
- Number: 2 identical heavy chains.
- Structure: Each heavy chain is made up of about 440-500 amino acids long.
- Domains: Each heavy chain contains one variable domain (VH) and three or four constant domains (CH1, CH2, CH3, and sometimes CH4, depending on the antibody class).
- Functions: Vh refers to the variable domain of the heavy chain, which contributes toward the site of antigen binding. The constant domains determine an antibody’s isotype and mediate effector functions, such as receptor binding on cells and complement activation.
2. Light Chains
- Number: 2 identical light chains.
- Structure: Each light chain contains about 214 amino acids.
- Domains: One variable domain is present in the light chain (VL) and one constant domain (CL).
- Function: The light chain variable domain, VL, is paired with the VH domain of the heavy chain in the antigen-binding site, while the constant domain, CL, provides structural stability.
3. Fab Region
- Composition: Composed of VL and CL domains of the light chain and VH and CH1 of the heavy chain.
- Function: This is the region where binding to the specific antigen occurs. Each antibody has identical two Fab regions, which can bind to two identical antigenic sites simultaneously.
4. Fc Region
- Composition: Formed by domains CH2 and CH3 of the heavy chains, and CH4 in some isotypes.
- Function: The effector functions of the antibody are mediated through the Fc region. This includes an interaction with immune cells via Fc receptors and activation of the complement system. In addition, this region also determines the half-life of the antibody in circulation. This region is important for linking antigen recognition with ways of immune response.
5. Hinge Region
- It is located between the Fab and Fc regions.
- Function: The hinge region gives flexibility to the antibody molecule, which allows the Fab regions to move and bind antigens more efficiently. Flexibility is also important for the interaction of an antibody with several antigens or closely spaced epitopes.
6. Antigen-Binding Site
- It is a composition of the hypervariable loops of the VH and VL domains, otherwise known as complementarity-determining regions (CDRs).
- Function: The antigen-binding site is where the antibody binds to its target antigen specifically. This specificity of binding and its affinity are governed by the precise shape and chemical nature of the CDRs.
7. Disulfide Bonds
Function: In the case of an antibody, there are disulfide bonds that link each heavy chain to each other and the light chains. They also stabilize the individual domains within the chains.
Monoclonal antibody development
There are key steps to be undertaken during the development of monoclonal antibodies to guarantee the efficacy and safety of the end product.
1. Target Identification
First is the identification of an appropriate target antigen that will be unique for the disease-causing agent or the cancer cells to minimize off-target effects.
2. Hybridoma Technology
The classical method of hybridoma technology involves the fusion of a B cell producing the desired antibody with myeloma a cancer cell and then develops a hybrid cell line that can be cultured indefinitely, producing large quantities of the antibody.
3. rDNA Technology
Modern approaches to generate mAbs are based on recombinant DNA technology. The genes encoding the antibody of interest are transferred into expression systems like mammalian cells, yeast, or bacteria.
4. Clinical Trials
Extensive clinical trials involving these mAbs are therefore conducted for safety, efficacy, and dosage. The phases of the clinical trials progress from small-scale safety studies to large-scale efficacy studies.
Also, Read| Immunoglobulin (Antibody) – Structure and Types
Applications of Monoclonal Antibodies
Cancer Treatment
- Checkpoint Inhibitors: Pembrolizumab and nivolumab are mAbs directed against immune checkpoints, which are cell surface molecules expressed on T-cells or tumor cells that can turn off T-cell activity following binding to their respective ligands.
- Targeted Therapy: Trastuzumab acts on HER2-positive breast cancer cells. Rituximab targets the CD20 molecule on the surface of B cells in certain lymphomas.
Autoimmune Diseases
- Rheumatoid Arthritis: Infliximab and adalimumab are included under the anti-TNF mAb category, corresponding to their brand names Remicade and Humira.
- Multiple sclerosis: Ocrelizumab targets CD20 on B cells, reducing the attack on nerve cells by the immune system.
Transplant Rejection
mAbs, like basiliximab, are directed against the interleukin-2 receptor on T cells to prevent an immune system attack on the transplanted organ.
References
- Goli, Naresh, et al. “Antibody-drug Conjugates (ADCs): Potent Biopharmaceuticals to Target Solid and Hematological Cancers- an Overview.” Journal of Drug Delivery Science and Technology, vol. 48, 2018, pp. 106-117, https://doi.org/10.1016/j.jddst.2018.08.022.
- Deb, Pran K., et al. “Biotechnology-Based Pharmaceutical Products.” Biomaterials and Bionanotechnology, 2018, pp. 153-189, https://doi.org/10.1016/B978-0-12-814427-5.00005-6.
- Weiner, Louis M., et al. “Monoclonal Antibodies: Versatile Platforms for Cancer Immunotherapy.” Nature Reviews Immunology, vol. 10, no. 5, 2010, pp. 317-327, https://doi.org/10.1038/nri2744.
Last Modified:
Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the third rank. Conducted various seminars and attended major Science conferences. Done 6 months of internship in ICMR – National Institute of Nutrition, Hyderabad. 5 years of tutoring experience.