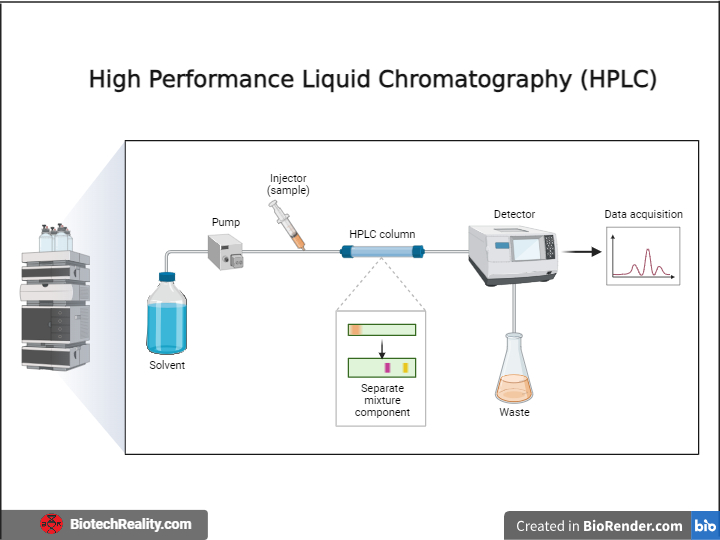
The powerful analytical method, high-performance liquid chromatography (HPLC), is employed in many scientific disciplines, including chemistry, pharmacology, biochemistry, and environmental studies.
It allows the precise and accurate separation, identification, and quantification of each component in a mixture. The fundamental concept behind HPLC originates from column chromatography, a process in which a mixture is passed through a stationary phase, and the various components separate out due to their multiple interactions with the stationary phase.
The high pressures used in HPLC, which typically range from 50 to 400 atmospheres, elevate this idea to a new level by accelerating the flow of the solvent and sample mixture across the column.
The HPLC system is made up of an assortment of vital components that cooperate to produce effective separation and analysis. These include a solvent reservoir, a pump to provide the high-pressure gradient, an injector to add the sample to the system, a column filled with a stationary phase, a detector to quantify the eluted components, and a data analysis system.
The various interactions between the components of the sample and the stationary phase inside the column are what drive the separation process in HPLC. The packing material, a finely divided solid substance that is commonly coated on a solid support or packed into a tube, is the stationary phase. This stationary phase can be made up of various substances, including silica, alumina, or custom phases made for particular separation tasks.
A further vital part of HPLC is the mobile phase or solvent. Based on the sample and the intended separation objectives, it is carefully selected.
The mobile phase is typically composed of an aqueous phase that has been buffered for pH control and an organic solvent mixture, such as acetonitrile or methanol. The pump regulates the composition of the mobile phase by producing a precise and repeatable gradient of solvents over time, enabling ideal separation conditions.
The sample is carried by the mobile phase and driven through the column after being inserted into the HPLC system via the injector. The different components experience varying degrees of retention as the mixture interacts with the stationary phase, leading to their separation down the column.
Depending on the type of analytes, the eluted components are next determined using a suitable detector, such as ultraviolet (UV) or visible light detectors, detectors of refractive index, or mass spectrometers. The data analysis system records the signal that the detector produces, which is proportional to the concentration of each component. Each component can be precisely identified and measured by
comparing its retention durations and peak areas to those of recognized standards or reference compounds. Over other separation methods, HPLC has many benefits. It offers great separation effectiveness, high sensitivity, and a variety of applications. Small molecules, peptides, proteins, nucleic acids, and complicated mixtures are among the many sample types it can handle.
Affinity chromatography, reversed-phase chromatography, normal-phase, ion exchange, and size-exclusion chromatography are just a few of the different separation methods that can be used with HPLC.
Also read: Next Generation Sequencing (NGS) and its application in genomics
Principle of HPLC
The separation of components in a mixture using a stationary phase and a mobile phase in a high-pressure system is the foundation of the High-Performance Liquid Chromatography (HPLC) method. In HPLC, a stationary phase-containing separation column is where the separation process takes place.
Small, porous particles, usually formed of granular material, make up the stationary phase. These particles give the sample components an immense amount of surface area for interaction. High pressure is used to drive the mobile phase, which is frequently a solvent or solvent mixture, through the separation column.
A pump is used to accomplish this, generating the required pressure to move the mobile phase through the system. The sample is carried by the mobile phase through the column, enabling the components to separate.
An injection system is utilized to introduce the sample into the mobile phase flow. This often involves a valve attached to a stainless steel capillary or short tube known as a sample loop. A syringe is used to inject the sample into the mobile phase flow, allowing it to combine with the mobile phase before entering into the separation column.
The various parts of the sample migrate across the column at various rates once they are inside. Each component interacts with the stationary phase in a distinct way, which results in differential migration. Stronger interactions with the stationary phase enable components to be maintained longer and migrate through the column more slowly than weaker interactions, which cause components to migrate through the column more quickly.
The various substances are then detected by an appropriate detector after passing through the separation column. UV/Visible light detectors, refractive index detectors, fluorescence detectors, and mass spectrometers are merely some of several detector types that can be utilized in HPLC.
A signal that is proportionate to the concentration of the separated components is produced by the detector. The computer’s HPLC software receives the detector signal and processes and analyzes it there. The program produces a chromatogram, a graphic representation of the detector signal as a function of time.
The separated components are identified and quantified by looking at the chromatogram, which shows peaks that correspond to them. The different substances in the sample can be identified by contrasting the retention durations of the chromatogram’s peaks with those of recognized standards or reference compounds.
The amount of each substance in the combination can be calculated using the peak areas or heights.

Instrumentation of HPLC
Pump
Pump systems were created as a result of HPLC advancement. The pump creates a flow of eluent from the solvent reservoir into the system and is positioned in the liquid chromatography system’s uppermost stream.
A “standard” requirement for pumps is the ability to generate high pressure, but it should also be able to deliver constant pressure under any circumstance as well as a predictable and repeatable flow rate. Reciprocating pumps, which are the majority of pumps used in present LC systems, move a motor-driven piston back and forth to create flow. This piston motion causes “pulses” to be produced.
Injector
In an HPLC system, the injector is in charge of adding the sample to the eluent flow for analysis. Syringe injections performed manually are appropriate for applications requiring low throughput or minimal work.
However, the recommended way for routine analysis is the employment of sampling loops with an autosampler system, which provides automatic and exact injections with improved efficiency and repeatability. The precise needs of the analysis and the desired level of automation determine the injection technique to be used.
Column
Inside the column is where the separation is done. Instead of glass columns, modern columns are frequently manufactured in stainless steel casing. As opposed to calcium carbonate, silica or polymer gels are the more common packing materials used.
Depending on the targeted separation and nature of the analytes, the eluent, or mobile phase, employed in HPLC may vary. Eluents come in a variety of acidic and basic solvents, providing a wide pH range and adaptability in the separation of various substances.
To accomplish the desired separation and maximize the resolution of the analytes, the eluent composition is carefully calibrated. Since stainless steel is resistant to a wide range of solvents, stainless steel is typically used for column housing.
The column and eluent both have a significant impact on the HPLC’s separation effectiveness and selectivity. The separation performance can be impacted by a number of variables, including column length, particle size, and dimensions. These settings are carefully selected to balance variables like resolution, analysis time, and system pressure while meeting the precise separation criteria.
Detector
Analyte separation is carried out inside the column, and the achieved separation is monitored by a detector. Based on different characteristics of the separated analytes, such as absorbance, refractive index, fluorescence, or mass, the detector detects and quantifies them. As a type of electronic signal, this discrepancy is tracked. There are numerous types of detectors available.
UV/Visible Light Detector: This detector is performed on the basis of absorbance spectroscopy. As the analytes pass through the detector cell, the absorbance of UV or visible light by them is measured. The absorbance signal is directly proportional to the concentration of the analyte.
Mass Spectrometer: Mass spectrometry (MS) detectors can provide structural information about the separated analytes and are highly sensitive and selective. The analytes are ionized, and the mass-to-charge ratio (m/z) of the resultant ions is measured. To identify and quantify analytes, mass spectrometers can be combined with HPLC and provide high-resolution detection.
Fluorescence detector: This detector monitors the fluorescence emitted by analytes that have fluorescence characteristics. It works by irradiating the analytes to a particular wavelength of light and observing the fluorescence that is produced at a different wavelength. For fluorescent substances, fluorescence detectors offer great sensitivity and selectivity.
Only a few of the detectors used in HPLC are shown in the list above. There are also other detector types that may be appropriate for particular applications or analyte classes, including refractive index detectors, electrochemical detectors, evaporative light-scattering detectors (ELSD), and conductivity detectors.
Recorder
Since the change in eluent is being detected by a detector as an electrical signal, it is still not apparent to the human eye. Pen (paper) chart recorders were widely utilized in earlier times. A computer-based data processor (integrator) is more popular these days.
These data processors offer greater flexibility, accuracy, and advanced data analysis capabilities compared to pen-chart recorders.
There are many different kinds of data processors, ranging from simple systems with an integrated printer and word processor to those with software that is specifically made for an LC system and includes features like peak-fitting, baseline correction, automatic concentration calculation, molecular weight determination, etc. in addition to data acquisition.
Degasser
In HPLC systems, the degasser is an essential part that removes dissolved gases from the eluent. The degasser efficiently removes gases like oxygen from the eluent stream by using a specific polymer membrane tubing with microscopic pores.
This membrane tubing is typically made from materials such as polytetrafluoroethylene (PTFE) or polyetheretherketone (PEEK). As a result, the eluent flow is stabilized, air bubbles are avoided, and a noise-free baseline is provided for chromatographic analysis.
By supplying a constant and consistent eluent composition, the degasser plays a crucial part in preserving the precision and dependability of HPLC tests.
Column Heater
A crucial part of HPLC systems, the column heater or column oven maintains a controlled environment for the separation column. It makes it possible to regulate temperature precisely, ensuring stable and repeatable temperature conditions for chromatographic analysis.
The best separation efficiency, selectivity, and resolution can be attained by managing the column temperature. For analyses that are sensitive to temperature changes or require elevated temperatures for greater resolution, the column heater is essential to getting reliable and precise HPLC findings.
Also Read: Random Amplified Polymorphic DNA (RAPD)
Types of HPLC
- Normal Phase HPLC: The stationary phase in normal phase HPLC is polar and often contains materials like silica. On the other hand, the mobile phase is non-polar. Water-sensitive substances, geometric isomers, cis-trans isomers, and chiral substances can all be separated using this method.
- Reverse Phase HPLC: A non-polar stationary phase is utilized in reverse phase HPLC, which is frequently packed with substances like C18. The mobile phase is commonly composed of water and an organic solvent that is miscible with water, such as acetonitrile or methanol. Because of its flexibility, reverse-phase HPLC can be used to separate polar, non-polar, ionizable, and ionic substances.
- Ion Exchange HPLC: Ion exchange HPLC uses an ionic-containing stationary phase. A buffer solution makes up the mobile phase, which enables the separation of anions and cations based on their interactions with the stationary phase’s charge. Ion exchange HPLC is frequently used in industries like pharmaceuticals and environmental studies and is useful for the analysis of charged species.
- Size Exclusion HPLC: Based on their size, HPLC separates molecules. An unevenly pored material is present in the stationary phase. In contrast to smaller molecules, which can diffuse into the pores and elute later, larger molecules are unable to penetrate the pores and elute first. exclude sizes Polymers, proteins, and other macromolecules can have different molecular weights or sizes, which can be identified via HPLC.
Advantages of HPLC:
- High Speed: Rapid analysis is possible using HPLC, making it appropriate for high-throughput applications. With improvements in instrumentation and column design, HPLC can offer quick separations, cutting down on analysis time and boosting lab productivity.
- Efficiency: The high peak performance of HPLC enables the resolution of complicated mixtures with exceptional separation efficiency. Modern HPLC columns with tiny particle sizes and optimum operating conditions produce superior peak morphologies and more effective separation of closely related substances.
- Accuracy: HPLC provides high precision in the identification and measurement of chemical components. It offers accurate measurements that can be relied upon, guaranteeing the precision of analytical results. This is essential in a variety of applications, including pharmaceutical analysis, environmental monitoring, and industrial product quality control.
- Versatility: HPLC is a flexible method that can be used with a variety of sample types, including small molecules, large biomolecules, polar and non-polar compounds, and ionizable substances.
- Accuracy: HPLC is incredibly accurate in determining the component concentrations in a sample. Even in matrices with complex compositions, it enables precise quantification of target substances at low levels. Applications include measuring medicinal potency, estimating impurity levels, and evaluating sample purity relying on this precision.
- Sensitivity: HPLC has a high sensitivity that makes it possible to detect and measure compounds at very low concentrations. The use of sensitive detectors, such as UV-Vis detectors, fluorescence detectors, and mass spectrometers, can improve sensitivity. This is helpful for environmental monitoring, pharmacokinetic investigations, and trace analyses.
- Automation and Robustness: HPLC systems are robust and capable of automation, which improves accuracy and reproducibility. Sample throughput is increased via automated sample handling, injection, and data gathering, which also reduces manual errors. Because of its durability, HPLC systems can tolerate a variety of sample loads and operating situations.
- Selectivity: The high selectivity offered by HPLC makes it possible to separate and analyze substances with comparable chemical properties. Even in complex matrices, selective separations can be accomplished by using a variety of column chemistries, stationary phases, and mobile phase compositions. In forensic analysis, food testing, and pharmaceutical analysis, selectivity is very crucial.
- Sample Compatibility: HPLC is capable of handling a wide variety of sample matrices, including liquids, solids, gases, and intricate combinations. Depending on the kind of sample, it enables flexibility in sample preparation methods and permits either direct injection or extraction-based sample preparation approaches.
Disadvantages of HPLC:
- Cost: Setting up and maintaining HPLC can be expensive. Specialized tools are required, such as high-pressure pumps, detectors, and columns. The overall cost to perform HPLC tests can also be impacted by the use of organic solvents and consumables.
- Complexity: The development of methods, the use of instruments, and the interpretation of data are all complex aspects of HPLC. It can be difficult and time-consuming to optimize separation conditions, choose suitable stationary phases and mobile phases, and troubleshoot potential problems, especially for complex samples.
- Sensitivity: HPLC is capable of achieving high sensitivity for a variety of substances, however, it might not be able to detect all substances. Due to inadequate response from the selected detector or interference from the sample matrix, some chemicals may have low detectability. More sensitive methods can be used to overcome this.
- Irreversible Adsorption: In some instances, some compounds may irreversibly adsorb to the packing material or stationary phase of a column, which will result in subpar recovery and detection. Incomplete separations or the disappearance of valuable analytes may arise from this. To overcome this restriction, special attention must be paid to choosing appropriate stationary phases and method optimization.
- Long Analysis Time: As compared to other chromatographic procedures, HPLC analysis occasionally needs longer run time periods. This is especially true when resolving closely eluting peaks or complex samples. Even while advances in instrument efficiency and column technology have significantly decreased analysis durations, high-throughput applications may still want to take this into account.
Applications of HPLC:
- Drug Analysis: HPLC is widely used for determining the identity, purity, and concentration of pharmaceuticals in pharmaceutical analysis. It is essential to the creation of pharmaceuticals, quality assurance, and formulation analysis.
- Analysis of Synthetic Polymers: HPLC is used to characterize synthetic polymers, including figuring out the composition, molecular weight distribution, and contaminants. This is crucial for sectors including textiles, coatings, and polymers.
- Pollutant Analysis in Environmental Analytics: HPLC is used to identify and measure contaminants in environmental samples such as soil, water, and air. It makes it possible to monitor pollutants including pesticides, drugs, and persistent organic pollutants.
- Drug Level Analysis in Biological Samples: Pharmacokinetic studies use HPLC to assess drug concentrations in biological samples such as blood, urine, and tissues. Understanding medication absorption, distribution, metabolism, and excretion is made easier by this.
- Isolation of value-added products; including natural products, pharmaceutical intermediates, and biomolecules, and their purification rely heavily on HPLC technology. It makes it possible to separate target molecules from intricate combinations.
- Purity and quality control of the product: HPLC is frequently used in industries to evaluate the purity and quality of the product. It guarantees that consumer goods, fine chemicals, and industrial chemicals all adhere to predetermined norms and legal criteria.
- Separation and Purification of Biopolymers: Proteins, enzymes, nucleic acids, and carbohydrates are among the many examples of biopolymers that can be separated and purified using HPLC. It makes it possible to isolate particular biomolecules for use in biotechnology applications or further investigation.
- Water purification: To remove impurities and clean up water sources, HPLC is used in water treatment operations. It enables the detection and quantification of organic molecules, byproducts of disinfection, and trace elements.
- HPLC can be used to pre-concentrate trace components in complicated samples, allowing for their identification and analysis at low concentrations. This is crucial for forensic, food, and environmental analyses.
Author: Ajmal Aseem
Published: 02-September-2023
Updated:
Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the third rank. Conducted various seminars and attended major Science conferences. Done 6 months of internship in ICMR – National Institute of Nutrition, Hyderabad. 5 years of tutoring experience.