Semaglutide has revolutionized the treatment of type 2 diabetes and obesity, making it one of the most influential therapeutic drugs in recent medical history. Semaglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist created by Novo Nordisk, addresses two of the most common metabolic diseases in the world by improving glycemic management and dramatically lowering body weight.
What is Semaglutide?
Human GLP-1 analogues, such as semaglutide, are synthetic forms of the hormone that the body naturally produces and are crucial for appetite and glucose metabolism. It acts similarly to natural GLP-1 by promoting insulin release, inhibiting glucagon secretion, delaying stomach emptying, and promoting satiety when administered as an oral tablet (brand name Rybelsus®) or as a weekly injection.
Following its original approval by the FDA in 2017 for type 2 diabetes (under the brand name Ozempic®), it received a historic approval in 2021 for the chronic control of weight in individuals who are obese or overweight (under the brand name Wegovy®). Thorough clinical trials that demonstrated significant results on blood sugar regulation and weight loss preceded their approvals.
Read More: Glycolysis – Pathway and Process Overview
Mechanism of Action of Semaglutide
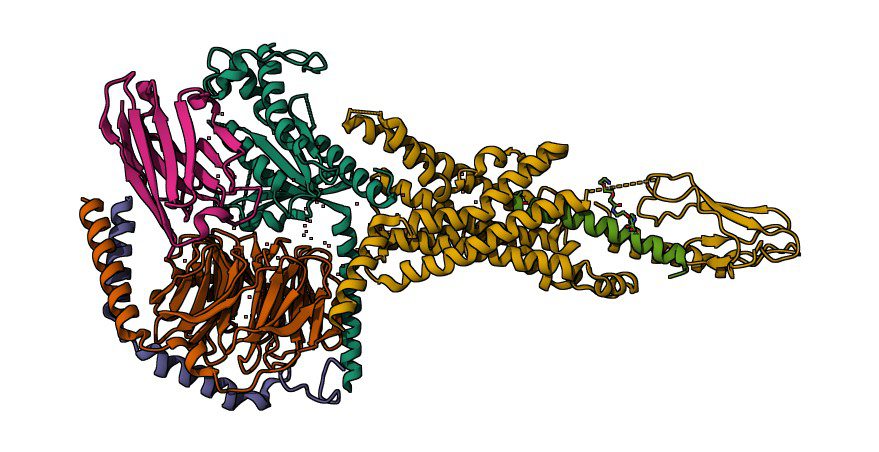
Semaglutide, a GLP-1 receptor agonist (GLP-1 RA), is intended to replicate the actions of glucagon-like peptide-1 (GLP-1), an endogenous incretin hormone. This hormone is essential for maintaining glucose homeostasis, controlling hunger, and maintaining gastrointestinal health. It is typically secreted by the gut in response to meal consumption.
The GLP-1 receptor, a G protein-coupled receptor (GPCR) expressed in the gastrointestinal tract, the cardiovascular system, the hypothalamus, and pancreatic islet cells, is activated by semaglutide.
Glucose-Dependent Insulin Secretion
- Semaglutide promotes glucose-dependent insulin production in the beta cells of the pancreas.
- This reduces the risk of hypoglycemia, which is a major benefit over sulfonylureas and insulin therapy, because insulin is only released when blood glucose levels are high.
- Through the PKA and Epac2 pathways, the activation of the GLP-1 receptor raises intracellular cyclic AMP (cAMP), which intensifies the insulin secretory response.
Suppression of Glucagon Secretion
- Semaglutide inhibits incorrect glucagon production in the pancreatic alpha cells in a glucose-dependent manner.
- When glucagon is not required, it inhibits hepatic gluconeogenesis and glycogenolysis, which lowers blood glucose levels by encouraging hepatic glucose synthesis.
- This also helps to keep postprandial and fasting glucose levels lower.
Read More: Trastuzumab: Targeted Therapy for HER2-Positive Cancers – Details, Mechanism, Usage and Side Effects
Summary of Mechanisms of Action of Semaglutide
| Target Site | Mechanism | Effect |
|---|---|---|
| Pancreatic β-cells | Glucose-dependent insulin secretion | Lowers blood glucose |
| Pancreatic α-cells | Inhibition of glucagon secretion | Reduces hepatic glucose output |
| GI Tract | Delayed gastric emptying | Reduces postprandial glucose, increases satiety |
| Hypothalamus (CNS) | Appetite suppression | Decreases caloric intake, promotes weight loss |
| Cardiovascular system | Anti-inflammatory, BP-lowering effects | Cardiovascular risk reduction |
| Kidneys | Natriuretic, hemodynamic improvements | Renal protection (under investigation) |
Safety Profile
Semaglutide is typically well tolerated, with gastrointestinal adverse effects nausea, vomiting, and diarrhea, being the most frequent. They are generally short-lived and can be controlled with a gradual increase in dose.
Market Impact and Future Outlook
Semaglutide has completely changed how two of the most debilitating illnesses are treated worldwide. Semaglutide may have a major long-term impact on patients and healthcare systems dealing with the skyrocketing costs of metabolic illnesses as obesity rates continue to rise.
Due to Novo Nordisk‘s success, demand is strong globally, and supply is limited. Although there are new competitors entering the market, such as Eli Lilly‘s tirzepatide (Mounjaro®/Zepbound), semaglutide remains the industry standard for the time being.
Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the third rank. Conducted various seminars and attended major Science conferences. Done 6 months of internship in ICMR – National Institute of Nutrition, Hyderabad. 5 years of tutoring experience.