Protein tangles known as amyloid-beta (A-beta) aggregates are most famously linked to neurodegenerative illnesses like Alzheimer’s. Nevertheless, despite its frequent attention, scientists have not been able to fully comprehend how A-beta assembles and disassembles.
The way A-beta behaves in a variety of environments, including the human brain, is elusive,
There’s an understanding of growth and decay that isn’t fully fleshed out.
Brian Sun
Their findings were published in the journal Nano Letters.
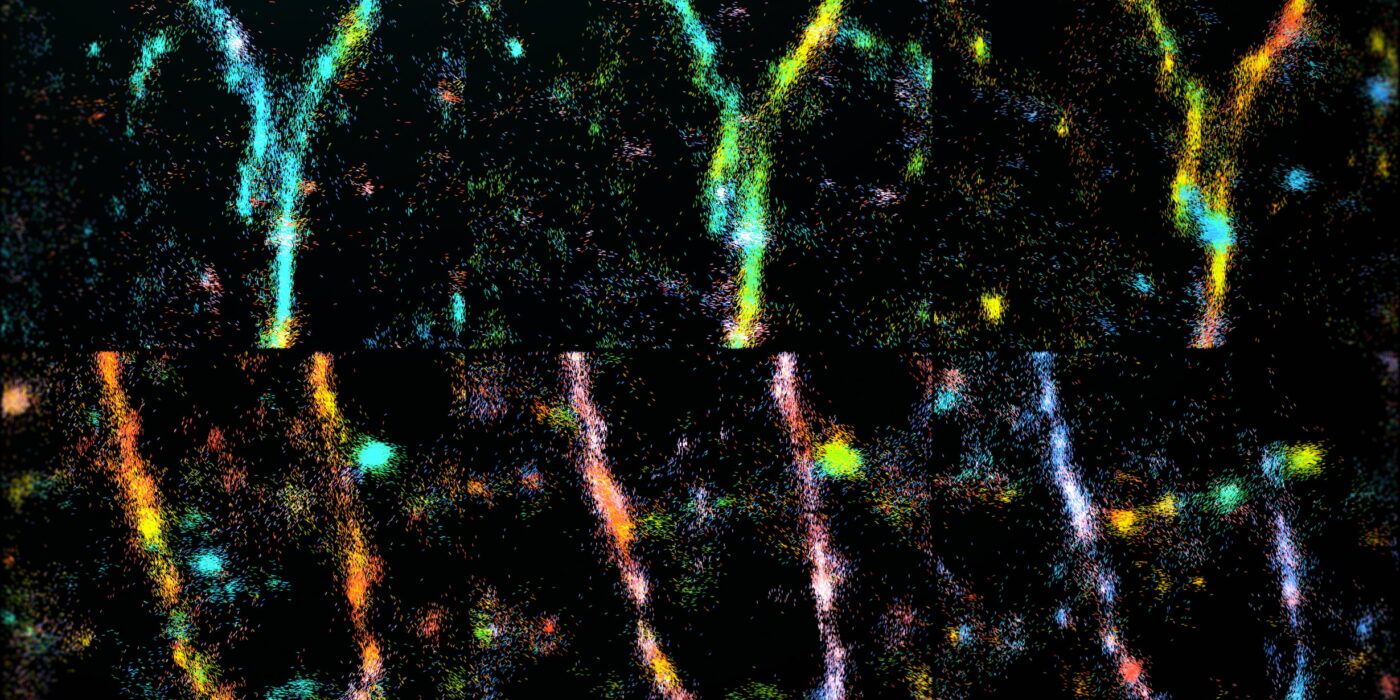
Sun and colleagues achieved a first: they could quantify the girders of the protein agglomeration, the underlying amyloid fibril beta-sheet assemblies, in real-time. Previous investigations using high-resolution microscopy have only obtained still images.
We wanted to look specifically at dynamics of the underlying structure of A-beta that could be responsible for the changes we’re seeing, not just changes in the overall shape.
Brian Sun
Lew compared it to Lego bricks, pointing out that while modern imaging technology can display an entire Lego construction, it cannot show you how the individual bricks are arranged.
The individual proteins are always changing in response to their environment,
It is like having certain Lego bricks causing other bricks to change their shape. The changing architecture of the proteins and the assembled aggregates together leads to the complexity of neurodegenerative disease.
Matthew Lew
Researchers can now discern orientation and other minute characteristics in the nanostructures of biological systems that were previously unseen thanks to a new imaging technology developed by the Lew lab. Their method, known as single-molecule orientation–-localization microscopy (SMOLM), visualises the sheets of peptides beneath Aβ42, a kind of A-beta peptide, by using light flashes from chemical probes.
They may examine the individual orientation of the underlying beta-sheets using SMOLM to determine how their arrangement connects to the general structure of the amyloid protein.
The Lew team discovered several surprises concealed in the amyloid-beta architecture after making some intuitive observations now that they can do these tests.
As anticipated, Aβ42 stable structures often have stable underlying beta-sheets, whereas structures that are developing have underlying beta-sheets that get more rigid and defined over time. Beta sheets in decaying structures are progressively less stiff and more disorganised. However, they also discovered other ways that A\42 may be updated.
There are multiple different ways for Aβ42 structures to remain stable, or grow and decay.
Brian Sun
Additionally, the researchers found that Aβ42 might behave unexpectedly in terms of growth and degradation. Aβ42, for instance, exhibits growth and decay behaviours that maintain the underlying structure. At times, the peptides merely pile on top of one another during growth, but the underlying beta-sheet orientations remain unchanged. In other instances, Aβ42 experiences “stable decay,” in which the converse occurs—that is, peptides go but the beta-sheet structure stays in place. Lastly, the beta-sheets of Aβ42 sometimes rearrange and shift their orientations without immediately affecting the overall shape. Future large-scale remodelling may be predisposed by this nanostructural reorganisation.
Because SMOLM can track Aβ42’s underlying organization and not just its shape, we can see different kinds of subtypes of remodeling that aren’t visible to diffraction-limited, non-orientation imaging modalities.
Brian Sun
Also, Read| Discovery of bacteria that can destroy Forever Chemicals
If all of this seems a little hazy, remember that this is only the initial attempt to even examine these dynamic nanoscale structures. The fact that Sun created this piece while balancing the COVID-19 lockdown constraints and his three-year undergraduate course load at WashU makes it even more noteworthy. It sets the path for him and other researchers to truly understand amyloid architecture.
Sun intends to develop nanoscale imaging systems and sensors that may disclose the underlying causes of illnesses that are challenging to cure throughout the graduate stage of his MD/PhD programme.
Sun acknowledges the rigorous training provided by McKelvey Engineering and the Lew lab for enabling this study and academic trajectory, as well as the MSTP for funding his post-graduation research.
Source: Washington University St. Louis – News Room
Journal Reference: Sun, Brian et al. “Single-Molecule Orientation Imaging Reveals the Nano-Architecture of Amyloid Fibrils Undergoing Growth and Decay.” Nano letters, 10.1021/acs.nanolett.4c01263. 3 Jun. 2024, DOI: 10.1021/acs.nanolett.4c01263.
Source: