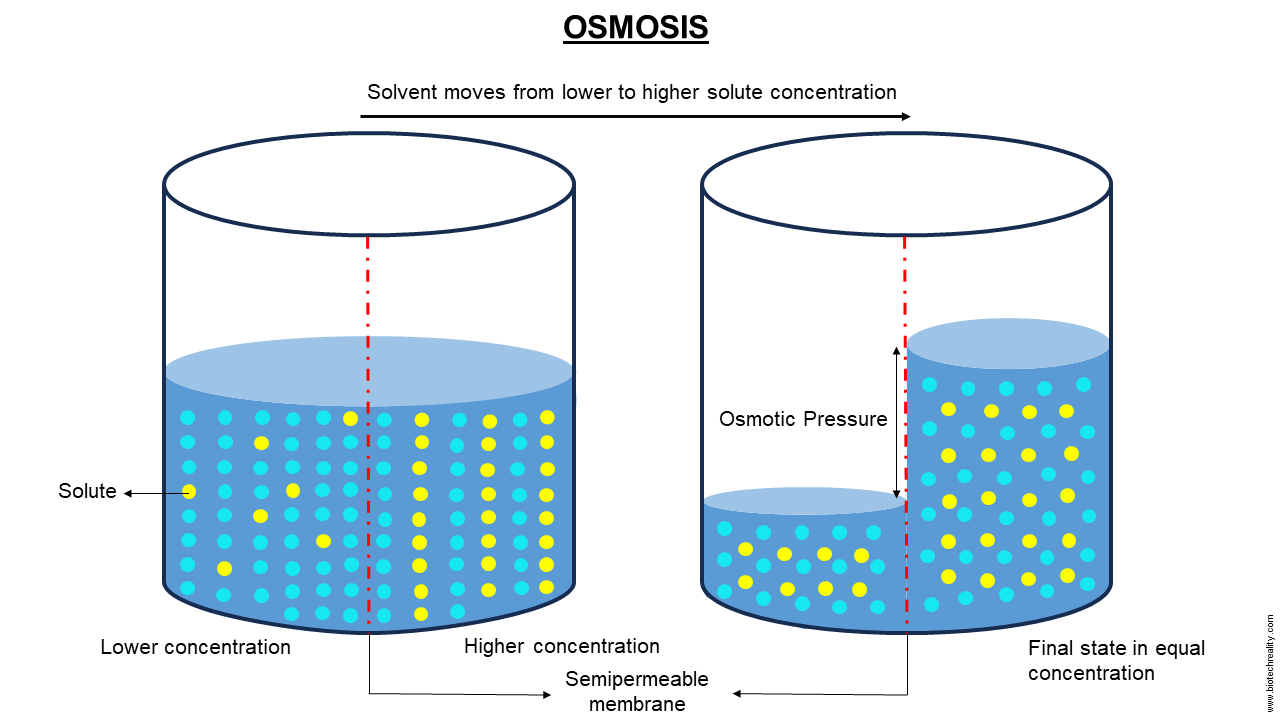
Osmosis is a mechanism in biology that includes the transfer of solvent molecules typically water from a region of lower solute concentration to a region of higher solute concentration across a selectively permeable membrane. This motion keeps on until equilibrium is achieved, at which point the concentration of solute on both sides of the membrane is the same. There is no need for energy input because the process is passive.1
The key factors that are necessary for osmosis are the semi-permeable membrane, concentration gradient, and equilibrium.
Principle
Osmosis is fundamentally the process of water molecules moving through a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. The osmotic pressure produced by the concentration difference is what propels this movement. Water flows net through the membrane because it permits water to pass through without obstructing the movement of solute particles. This process continues until equilibrium is achieved.
Role in Biological Functioning
Osmosis is essential for maintaining the structural integrity and functional integrity of cells. A regulated osmotic environment is essential in animal cells to avoid excessive water absorption, which can cause swelling and eventual rupture of the cell. However, in terms of turgor pressure, plant cells—which have inflexible cell walls—benefit from osmosis. Water enters the cell by osmosis, which keeps the cell structurally intact and keeps the plant from withering.
Types of Osmotic Solutions
The relative concentrations of solutes and solvents on each side of a semi-permeable membrane are used to classify osmotic solutions.
Isotonic Solution
When the solute concentration is the same on both sides of the membrane, the solution is said to be isotonic.
Hypotonic Solution
A solution that has a lower solute concentration outside the cell than within is said to be hypotonic.
Hypertonic Solution
A solution that has a larger solute concentration outside the cell than within is said to be hypertonic.
Requirements for Osmosis
Semipermeable Membrane
Osmosis takes place across a semi-permeable membrane that permits solvent molecules typically water to flow through while limiting solute particle mobility due to factors including size and charge.
Osmotic Pressure
Osmotic pressure is the result of solvent molecules moving around. The force that water passes through the membrane is determined by this pressure, which is proportionate to the concentration gradient.
Concentration Gradient
A concentration gradient, or the variation in solute concentration between the two sides of the membrane, is what propels osmosis. From a region with a lower solute concentration to one with a greater solute concentration, the solvent molecules migrate.
Equilibrium
Osmosis continues until equilibrium is reached, at which point the solute concentration is the same on both membrane surfaces. Even if individual water molecules are still travelling across the membrane in both directions, there is now no net flow of water.
Osmotic Pressure
A key idea in the study of osmosis is osmotic pressure, which describes the force that solvent molecules typically apply as they migrate over a semi-permeable membrane from a region with a lower solute concentration to one with a greater solute concentration. The solvent molecules’ propensity to migrate and balance the solute content on both sides of the membrane is the factor that causes this pressure.
The pressure units used to measure osmotic pressure are usually pascals (Pa) or atmospheres (atm). The Van der Hoff equation provides the following expression for osmotic pressure (π):
π=iMRT
M is the molarity of the solution, R is the ideal gas constant, and T is the absolute temperature. In practical applications, osmotic pressure is used to purify water through procedures like reverse osmosis. It is also pertinent in medical settings, particularly in dialysis, when waste items are eliminated from the blood by the use of osmotic pressure.
Also Read| Mitosis: Definition, Stages, Mechanism of Cell Division, and Diagrams
Applications of Osmosis
Biological
1. Transportation within cells
Diffusion and active transport are two more cellular transport processes that are closely related to osmosis. When combined, these mechanisms guarantee the proper distribution of necessary materials like ions, nutrients, and waste products both inside and between cells.
2. Osmolarity of blood and kidneys
The control of blood osmolarity depends on osmosis. Osmosis, for instance, is used by the kidneys to reabsorb water only when necessary and to keep the body’s water balance. The nephrons, which are responsible for producing urine, carry out this process by reabsorbing water into circulation and adjusting solute concentrations.
Normal Life
1. Preservation of Food
Food preservation methods like pickling and brining make use of osmosis. Fruits, vegetables, or meats are preserved by drawing water out of microorganisms’ cells and stopping their development in high-concentration salt solutions.
2. Dialysis (Medical)
Osmosis has applications in medicine, particularly in dialysis technology. Dialysis devices replicate the natural processes that occur in healthy kidneys by using osmosis and diffusion to remove waste materials from the blood of patients suffering from kidney failure.
3. Laboratories
In laboratories, this method is used for the development of membranes as well as the cell cultures. This method also supports water purification by reverse osmosis.
Also Read| HIV – Human Immunodeficiency Virus | Details
FAQ
Osmosis is a biological process in which molecules of a solvent, usually water, move from a region of lower solute concentration to a region of greater solute concentration across a semi-permeable membrane. Water moves in this manner until equilibrium is attained, at which point the concentration of solute on both sides of the membrane is the same.
Endosmosis: The solvent molecules rush into the cell and become turgid when the cell is placed in a hypotonic solution.
Exosmosis: The shrinkage of the cell happens when the cell is placed in a hypertonic solution so that the solvent molecules move out of the cell.
The principle of osmosis is applied to the dialysis technology. This is used for the purification of the blood in with patients kidney failure.
A semi-permeable membrane is used in the reverse osmosis process to extract ions, molecules, and bigger particles from water. It forces water across the membrane against its natural osmotic flow in water purification systems to generate clean and pure water.
Water travels across a semi-permeable membrane during the process of forward osmosis from a region with a lower solute concentration to one with a higher solute concentration. This happens naturally without the need for external pressure, and it may find use in desalination and water treatment.
A condition known as osmotic lysis happens when a cell absorbs too much water in a hypotonic solution, which can lead to the cell swelling and perhaps rupturing. This is pertinent when considering cell biology and how crucial it is to preserve appropriate osmotic equilibrium.
Osmoregulation is the process by which an organism regulates its solute and water concentrations in order to preserve homeostasis. It involves the water balance and ion concentration adjustments in cells and tissues, among other systems that control osmotic pressure.
Author: Achuth B S
Last Updated:
Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. M.Sc. Biotechnology from the University of Kerala. Attended certificate course in Artificial Intelligence for Everyone from Deeplearning.AI, Influenza Prevention and Control from World Health Organization. Attended workshops related to Bioinformatics at the University of Kerala. 3 years of experience in website management. Experience in WordPress, Blogger, Google Analytics, and Google Search Console.