Sanger sequencing, also known as the dideoxy chain-termination method, is one of the cornerstone techniques for determining the exact nucleotide sequence of DNA. Introduced by Frederick Sanger and co-workers in 1977, it has been an important tool for genetic research, especially for determining entire genomes, such as in the Human Genome Project. Although new techniques, such as NGS, have emerged, Sanger sequencing is still considered a gold standard for smaller-scale projects, validation studies, and high-accuracy applications.
Principle of Sanger Sequencing
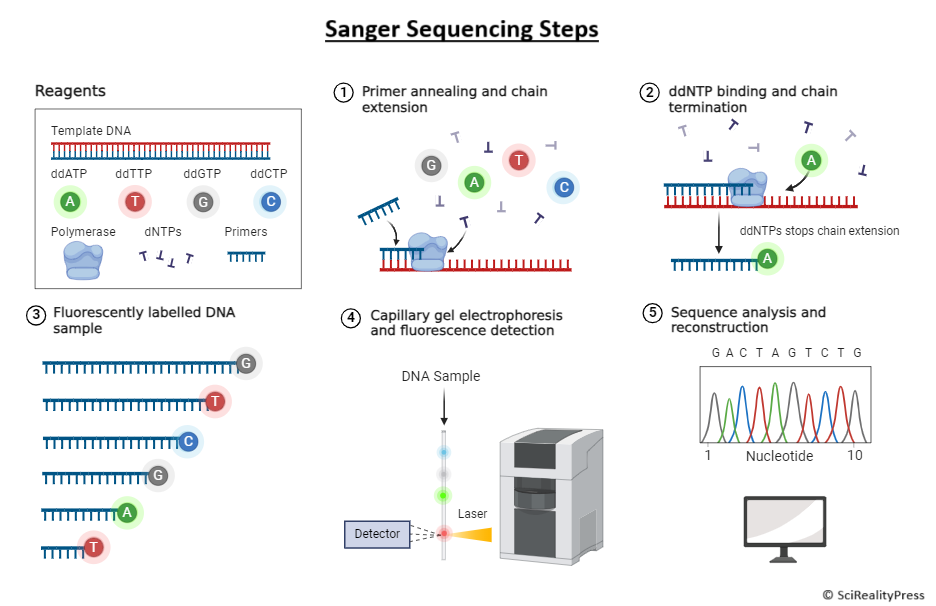
This is achieved through the selective incorporation of chain-terminating dideoxynucleotides, known as ddNTPs, into DNA synthesis. The ddNTPs lack a 3′-hydroxyl group and thus cannot form a phosphodiester bond with the next nucleotide and essentially terminate DNA strand elongation. The incorporation of those terminators at random positions along the elongating DNA allows the sequence to be determined based on the lengths of those terminated fragments.
Components in Sanger Sequencing
- DNA Template: The single-stranded DNA to be sequenced.
- Primer: A short single-stranded oligonucleotide complementary to the target sequence. It is a primer for the elongation by DNA polymerase.
- DNA Polymerase: This enzyme synthesizes new DNA strands by adding nucleotides to a primer.
- Deoxynucleotides (dNTPs): The nucleotides that are added in the synthesis process of DNA and are A, T, C, or G in the normal form.
- Dideoxynucleotides (ddNTPs): Modified nucleotides (ddATP, ddTTP, ddCTP, and ddGTP) that interrupt DNA strand synthesis because, upon incorporation, they lack the 3′-hydroxyl (-OH) group.
Sanger Sequencing Procedure
- Preparation of Reaction Mixture: Pool the DNA template, primer, dNTPs, ddNTPs, and DNA polymerase together in a reaction tube. The concentration of ddNTPs is much lower than that of dNTPs so that most chain elongations proceed normally but terminate at times during the incorporation of a ddNTP.
- Chain Elongation and Termination: Once initiated, DNA polymerase continuously adds dNTPs to the ever-growing chain. Incorporation of a ddNTP instead of its corresponding dNTP stops elongation. This means that fragments of DNA of different lengths have been produced, each terminating at a specific nucleotide.
- Gel Electrophoresis: Gel Electrophoresis separates DNA fragments by size. In modern automated sequencing, the ddNTPs are labeled with different fluorescent dyes-one per nucleotide-and an optical detector reads the fluorescence as each fragment passes through the gel, so the sequence can be read directly.
- Data Analysis: a chromatogram or electropherogram is produced. This graph represents the DNA sequence as color peaks for each one of the four nucleotides. The order of the peak can then be interpreted for the sequence.
Radioactive and Fluorescent Labelling
Primarily, Sanger sequencing required a method using radioactive labeling of the ddNTPs for detection. It entailed a sequence of running four separate reactions-one for each nucleotide-and each reaction has to undergo gel electrophoresis. With time, modern Sanger sequencing used fluorescent labeling, wherein all four reactions could be done in one tube. Each of the ddNTP was tagged with a different fluorescent dye, which was detected during electrophoresis using a laser, making the process more efficient and automated.
Next Generation Sequencing Comparison
Also Read| Next Generation Sequencing (NGS) and its application in genomics
- Throughput: NGS technologies can sequence millions of fragments in parallel, but Sanger sequencing sequences one fragment at a time.
- Cost: For large-scale studies, NGS is significantly cheaper compared to Sanger sequencing. For low-throughput projects, though, it is relatively more inexpensive to conduct using Sanger.
- Read Length: Sanger sequencing produces more extended reads-about 1000 base pairs-up to NGS yields somewhat shorter reads-100–300 base pairs.
- Accuracy: While the NGS technologies improved with regards to correcting errors over time, the Sanger sequencing had a higher accuracy level for specific areas.
Advantages of Sanger Sequencing
- High Accuracy: Sequences gained from Sanger sequencing have an error rate of around 1 in 100 000 bases, making it highly accurate for sequence determination.
- Constantly Reliable for Small Genomic Regions: It is very useful for the sequencing of small DNA fragments (upto about 1000 base pairs), such as individual genes or plasmids.
- Ease of Interpretation: The results (chromatograms) are easy to interpret, especially for clinical applications where precision is the aim.
- Longer read lengths: This can produce longer read lengths compared to most methods of NGS, about 700-1000 base pairs, that will be useful for the resolution of repetitive sequences or ambiguous regions.
Disadvantages of Sanger Sequencing
- Low Throughput: Sanger sequencing is labor-intensive and slow in large projects. It requires time to sequence one fragment of DNA at a time; this is inefficient when compared to NGS technologies, which can sequence millions of fragments at the same time.
- Expensive for Large Projects: Although it’s cheaper for relatively small projects, the cost per unit price of Sanger sequencing makes it unaffordable for larger genomes because of its low throughput.
- Low Scalability: NGS technologies are appropriate when high-scale sequencing is needed, for instance in whole-genome sequencing or transcriptome analysis where much larger datasets are accommodated.
Applications
- Small-scale Sequencing: It is ideal for small-scale investigations, sequencing of single genes for cloning experiments, and verification of sequences derived from other sequencing techniques.
- Diagnostic Tests at the Clinical Level: In clinic rooms, it is typically used to search for genetic mutations in patients, particularly for diseases that are caused by genetic mutations, since it is highly reproducible and stable in its testing.
- Mutation Detection: It is used to validate the existence of SNPs, insertions, and deletions and all the types of mutations.
- Haplotype Analysis: Because of this, haplotypes and alleles can also be done through Sanger sequencing, which is very crucial in research population genetics and evolutionary biology.
- Validation of NGS Data: Although next-generation sequencing methods are indeed much faster and cheaper for large-scale sequencing, this technology finds validity in specific regions of the genome coming out from NGS through Sanger sequencing because this method has higher accuracy for detecting low-frequency mutations.
Image Source: Created in BioRender.com
Last Modified:
Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the third rank. Conducted various seminars and attended major Science conferences. Done 6 months of internship in ICMR – National Institute of Nutrition, Hyderabad. 5 years of tutoring experience.