Immunoglobulins
The glycoproteins produced by B-lymphocytes effector cells or plasma cells against specific antigens are known as Immunoglobulins or Antibodies. Antibodies are made up of multiple immunoglobulin domains that are composed of a polypeptide chain that folds into a series of antiparallel β-pleated strands and these strands are arranged into pair of β sheets within each domain to form a compact tertiary domain. The two β-pleated strands in each domain are held together by the hydrophobic interaction and disulphide bonds.
Structure
The basic structure of antibodies is the same in that they have four polypeptide chains, of which two are light (L) chains of size approximately 25 KDa each and two are heavy (H) chains of size approximately 50 kDa. Two heavy chains are connected to each other by two disulphide bonds and light chains are also connected to each other by disulphide bonds as each light chain is connected to its heavy chains by disulphide bonds and by non-covalent interactions between VH and VL domains and CH1 and CL domains.

Based on the basic structure of antibodies, the variable region of both heavy (VH) and light chains (VL) are regions where the antigen binds and are called Antigen binding fragments or Fab and two Fab regions are there so are termed F(ab)2 and the remaining region of the antibody is call the Fc region or Fragment crystallizable. In the variable region, there are some highly variable regions that are called the complementarity determining region (CDRs) that specifically determine the Ag-Ab complementarity and the less variable regions in an immunoglobulin are called the constant region.
The constant regions in the heavy chains are CH1, CH2, and CH3 and those of the variable chain are CL. The space between CH1 and CH2 is known as the hinge region, which is highly flexible. The carbohydrate part of an immunoglobulin is present in the CH2.
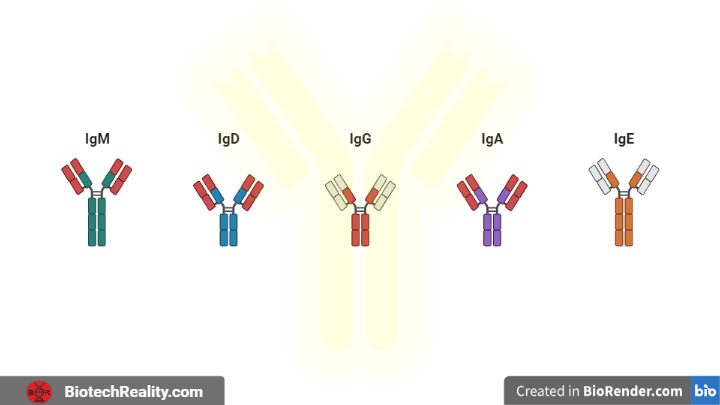
Classes of immunoglobulins based on the heavy chain isotypes
There are two major classes of antibody light chains which are kappa (κ) and lambda (λ) and those are constant regions there are five major classes of heavy chains μ (mu), δ (delta), γ (gamma), ε (epsilon), and α (alpha) and are in constant regions and they are referred to as an isotype of antibody heavy chain that determines the different antibody class.
- IgG – γ
- IgM – μ
- IgA – α
- IgD – δ
- IgE – ε
In these IgM and IgD are membrane-bound and others are secreted in form.
1. IgG

- IgG is the antibody that is present in serum about 75% and has a half-life of more than 23days
- Is present in a monomeric form
- There are four different types of IgG1, IgG2, IgG3 and IgG4 differences in the number of interchain disulphide bonds
- Involved in passive acquired natural immunity as it is the only immunoglobulin that can cross the placenta
- Second released antibody when there is an infection
- Involved in opsonisation, i.e., they act as opsonin
- Helps in complement activation
2. IgM

- Only 5-10% is present in serum and is short-living
- The presence of IgM indicates a recent infection
- It is present in Pentameric form
- Five immunoglobulin chains are clipped together to form the pentameric IgM and the clip is called J-chain or joining the chain, an extra polypeptide
- It is the first produced immunoglobulin in a newborn baby (neonatal)
- IgM has a greater number of paratopes (antigen binding regions present in an antibody) and, they have more valency i.e., they can bind with many epitopes (antigenic regions that bind with the antibody)
- Involved in complement activation
3. IgA

- IgA, an antibody that presents about 15% of serum
- It is present in dimeric form, in which two immunoglobulin chains are clipped with a J-chain
- It is present in breast milk as well as found in saliva, tears, mucous membranes, etc.
- Provides defence against bacterial and viral infections and protects the newborn
- It consists of a secretory chain when it is present in milk but it is gained after it is produced by the plasma cells.

4. IgD

- Present in monomeric form, about only 1% seen in the serum
- Involved in chemotaxis (signalling via recognising chemicals)
- Controls food allergens along with IgE
- Involved in TH2 cell-mediated immunity
- Recognises soluble antigen
5. IgE

- Seen a very less amount in the serum, 0.001-0.2%
- Involved in allergy and parasitic infection
- Mediate type 1 immediate hypersensitivity or atopy
- Can bind with the Fc receptors of mast cells and basophils
References: Kuby Immunology 7th Edition
Last Updated:
Qualified CSIR - National Eligibility Test (NET), eligible for Assistant Professorship in any Indian university. Graduated from the University of Kerala with B.Sc. Botany and Biotechnology. Attained Post-Graduation in Biotechnology from the Kerala University of Fisheries and Ocean Science (KUFOS) with the fourth rank. Conducted various seminars and presentations. Experienced in ELISA, Blotting, and other Good Laboratory Practices. Attended a certificate course in Patent Analytics. Done 6 months of internship in ICMR - Vector Control Research Center, Puducherry. 3 years of tutoring experience.